- Ex 5A, P. 151-155, Models of the Atom
Parts of the Atom
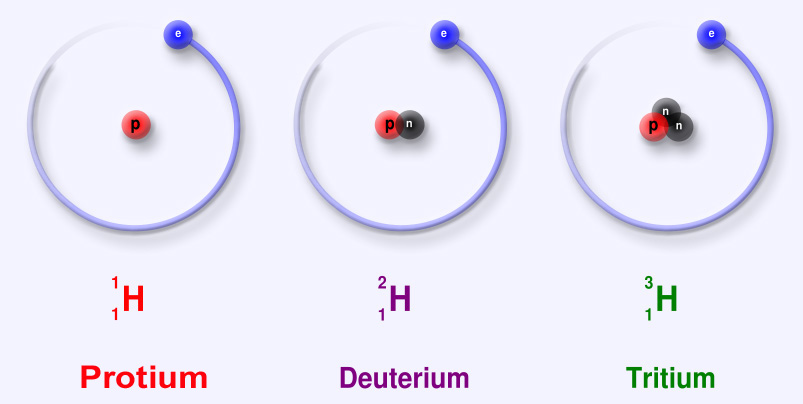
Isotopes
Isotopes are versions of an atom or an element that have the same number of protons, but different numbers of neutrons
Radioactive Decay
Alpha, Beta & Gamma Radiation
- Alpha particles are emitted by a larger nucleus that has too many Protons
- Beta particles are emitted by a nucleus that has too many Neutrons
- Gamma photons are emitted by a nucleus that has too much Energy (is in an exited state)
Alpha Decay
Beta Decay
Radiation
Types of Radiation
Half-Life
Half-Life Explanation
Half-Life Calculations
Half-Life Formula
A = Ao(½)n
A: Amount of radioactive substance now present
Ao: Amount of radioactive substance originally present
n: Number of Half-Lives that have past
If you can understand the maths for using the formula above, you will be able to pinpoint your answers instead of estimating them using a graph.
You will need to be able to use the 3rd Log Law to solve for half lives.

No comments:
Post a Comment